PharmaShots Weekly Snapshots (October 23–27, 2023)
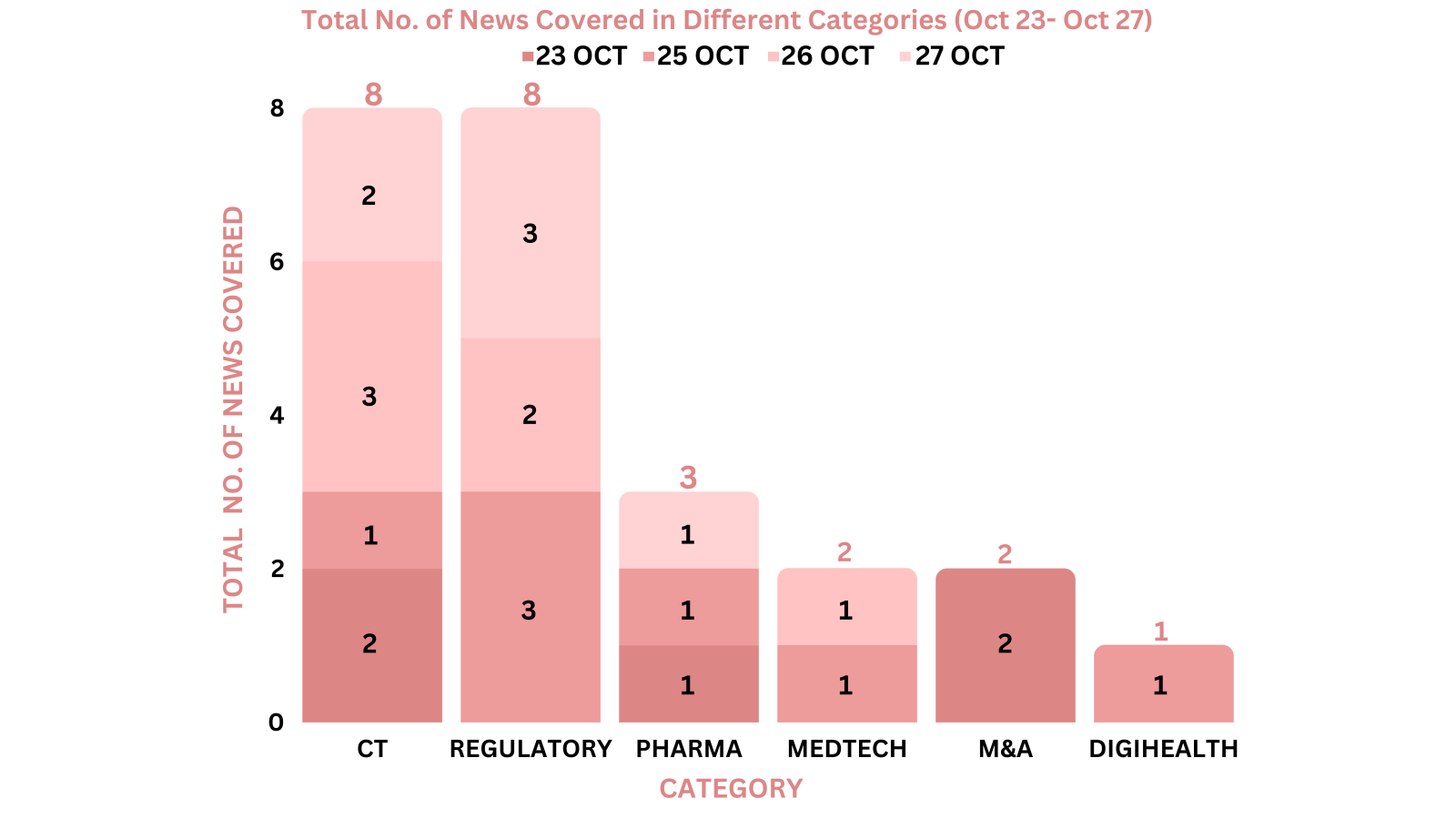
This week PharmaShots’ news was all about the updates on clinical trials, regulatory, pharma, M&A, MedTech and DigiHealth. Check out our full report below:

The US FDA approved Celltrion's Zymfentra (Infliximab-Dyyb) for the treatment of inflammatory bowel disease
Read more: Celltrion
The US FDA granted BMS's BMS-986278 a break through designation for the treatment of pulmonary and progressive pulmonary fibrosis (IPF & PPF)
Read more: BMS
The US FDA granted approval to Servier’s Tibsovo for the treatment of Myelodysplastic Syndromes (MDS)
Read more: Servier
ViiV Healthcare's Vocabria in combination with Rekambys receives the NMPA’s approval as an HIV-1 injectable treatment
Read more: ViiV Healthcare
The US FDA has approved Eli Lilly’ Omvoh (mirikizumab-mrkz) for adults with moderately to severely active ulcerative colitis. based on the (LUCENT) program incl. 2 P-III trials i.e., one 12wk. induction study (UC-1) and one 40wk. maintenance study (UC-2) for 52wks.
Read more: Eli Lilly
The US FDA has approved Genentech’s Vabysmo (faricimab-svoa) for retinal vein occlusion, based on the P-III (BALATON) for branch retinal vein occlusion and (COMINO) studies for central retinal or hemiretinal vein occlusion
Read more: Genentech
The US FDA has accepted the IND application to initiate a P-I trial of Mustang Bio’ MB-109, a novel combination of MB-101 and MB-108 in adult patients with recurrent GBM and high-grade astrocytomas
Read more: Mustang Bio
Amgen presents the data for Lumakras and Vectibix in P-III clinical trial for the treatment of metastatic colorectal cancer at ESMO 2023
Read more: Amgen
The results from the P-III clinical trials evaluating TrenibotulinumtoxinE (BoNT/E) for treatment of glabellar lines were reported by Allergan and AbbVie
Read more: Allergan and AbbVie
Revolo Biotherapeutics reports the P-IIa trial results for IRL201104 in seasonal allergic rhinitis
Read more: Revolo Biotherapeutics
Imugene reports the initiation of the P-I (OASIS) study for onCARlytics in solid tumor patients
Read more: Imugene
The results from the P-III trial evaluating Leqembi as subcutaneous dosage form for Alzheimer’s Disease were presented by Eisai and BioArctic at CTAD 2023
Read more: Eisai and BioArctic
MaaT Pharma received positive opinion from DSMB for MaaT013 in P-III trial to treat graft versus host disease (GvHD)
Read more: MaaT Pharma
Pfizer and BioNTech highlighted P-I/II study result of mRNA-based combination vaccine program against influenza and COVID-19 which showed robust immune responses to influenza A, influenza B, and SARS-CoV-2 strains
Read more: Pfizer and BioNTech
BioCryst initiated the patient enrolment in proof-of-concept trial of BCX10013 for the treatment of complement-mediated diseases
Read more: BioCryst
The US FDA approved Medinol’s EluNIR-PERL drug-eluting coronary stent system to treat coronary artery disease
Read more: Medinol
Fresenius Medical Care recalls few models of haemodialysis machines for potential exposure to toxic substances "IN" the US
Read more: Fresenius Medical Care
PENTAX Medical INSPIRA (EPK-i8020c) and i20c Endoscope Series by PENTAX Medical debuted in USA at ACG 2023
Read more: PENTAX Medical

Alaya.bio acquired Ixaka France to strengthen its novel in vivo CAR-T immunotherapy platform
Read more: Alaya.bio and Ixaka France
Roche acquires Telavant along with its asset RVT-3101 from Roivant to treat inflammatory bowel disease
Read more: Roche and Telavant

Together with AstraZeneca and Everton, LumiraDx intends to launch a heart and lung screening center for its NT-proBNP test in England
Read more: AstraZeneca and Everton

GSK entered into a license agreement with Hansoh for HS-20089 to treat gynecologic cancer
Read more: GSK and Hansoh
Eli Lilly signed a collaboration and licence agreement with Elektrofi to develop patient-centered subcutaneous therapies
Read more: Eli Lilly and Elektrofi
Mithra & Searchlight signed a license and supply agreement for Donesta in Canada
Read more: Mithra & Searchlight
Related Post: PharmaShots Weekly Snapshots (October 16–20, 2023)
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.